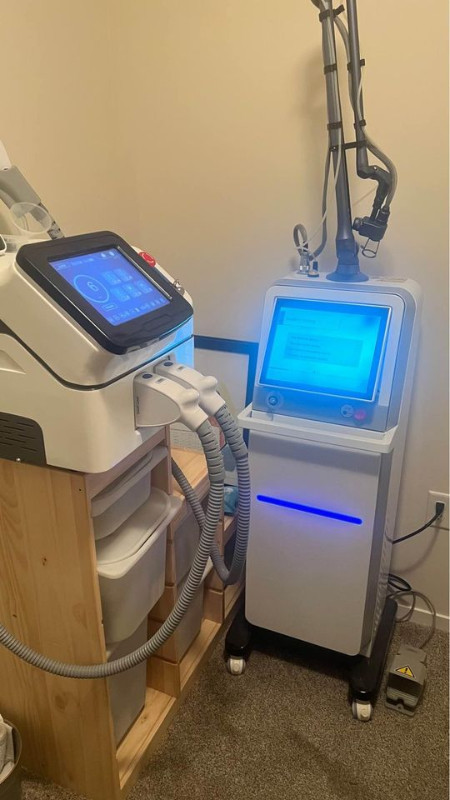
SPA Laser/IPL Bundle - Financing Available



- Condition
- Used - Like new
Description
SPA Laser Bundle
Financing Available From Greg at LeaseLine https://leaseline.com/ Greg@leaseLine.com
Getting out of business.
IPL Laser Triple Wave Diode Laser Fractional Laser
Both Diode and IPL have Frosted Tips to minimize swelling and patient comfort
Every Laser You Need
Must be bought together for discount. I’m selling at a loss.
Amazing startup deal for a startup home SPA or retail location. These are recognized as safe by health Canada. See pictures and txt below.
First come first serve
What is ISO13485?
ISO 13485 is an international standard that outlines the requirements for a comprehensive quality management system for the design and manufacture of medical devices. This standard focuses on the safety and effectiveness of medical devices, and requires organizations to demonstrate their ability to consistently produce medical devices that meet customer and regulatory requirements.
In Canada, laser diode machines used for medical purposes must comply with ISO 13485 in order to be legally sold and operated. This means that the manufacturer of the laser diode machine must have a quality management system in place that meets the requirements of ISO 13485, and must be able to demonstrate its compliance to Health Canada during the regulatory approval process.
In general, ISO 13485 is an important standard for ensuring the safety and effectiveness of medical devices, including laser diode machines used for hair removal and other skin treatments. By complying with this standard, manufacturers can provide customers with confidence in the quality and reliability of their products.
Device is Certified under: ISO 13485 -- International Agreement to provide medical devices and related services that consistently meet customer and applicable regulatory requirements. Health Canada will only accept quality system certificates that have been issued by special third-party auditing organizations called Canadian Medical Devices Conformity Assessment System (CMDCAS) recognized registrars. The Medical Devices Regulations do not require importers or distributors of medical devices to have a registered quality system.
As stated on List of Registrars Recognized by Health Canada under section 32.1 of the Medical Devices Regulations (MDR) specifies which ISO registrars are recognized by Health Canada as to certify these kind of medical grade devices. https://www.canada.ca/en/health-canada/services/drugs-health-products/medical-devices/quality-systems-13485/list-registrars-recognized-under-section-32-1-medical-devices-regulations.html
TUV Rheinland of North America, Inc. is on this list and on the North American Website lists our machines
Follow https://www.tuv.com/
While at the TUV Rheinland Main Page, Select The Third Blue Box Mid Right 'Find Detailed Information On Test Marks, IDs, products and companies on Certipedia' https://www.certipedia.com/companies/585868/system_certificates?locale=en
Inside The ID field enter 9000012975
Results: Management Systems EN ISO 13485 and MDD Annex II excl. 4 Certificate Holder Beijing Stelle Laser Technology Co.,Ltd. Test Mark Number 9000012975
EN ISO 13485:Design and Development, Manufacture and Distribution of Dermatological Diode Laser Systems MDD Annex II excl. 4:Dermatological Diode Laser Systems https://www.certipedia.com/quality_marks/9000012975?locale=en&certificate_number=HD+2264988-1
See the Certificate Number 2264988-1
See Screenshots of The EN ISO 13485 Certificate And MDD Annex II[Medical Device Directive Declaration Of Conformity]
Medical CE -- CE marking is the medical device manufacturer's claim that a product meets the General Safety and Performance Requirements (GSPR) of all relevant European Medical Device Regulations and is a legal requirement to place a device on the market in the European Union
RoHS -- Restriction of Hazardous Substances in Electrical and Electronic Equipment (RoHS) EU rules restricting the use of hazardous substances in electrical and electronic equipment to protect the environment and public health.
A FDA Premarket Certification Process[K212478] as a Clinical Dermatological Diode Laser System under regulation number 878-4810 is in the final stage and this machine will be grandfathered as fully FDA Approved.
Above is for the Diode / IPL
Below is for the Fractional Laser
https://www.certipedia.com/certificates/60141248?locale=en
https://www.certipedia.com/quality_marks/9000008262?locale=en&certificate_number=SX+2059222-1%2F02
https://www.accessdata.fda.gov/cdrh_docs/pdf16/K162398.pdf